
All categories
Featured selections
Trade Assurance
Buyer Central
Help Center
Get the app
Become a supplier

(187805 products available)





































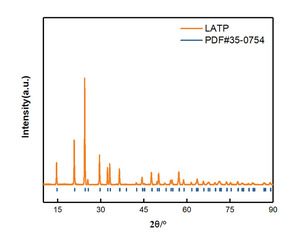
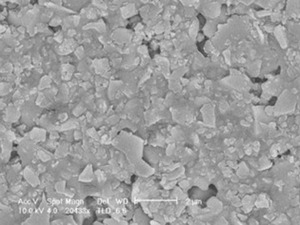







Ceramic electrolytes play a fundamental role in solid oxide fuel cells (SOFCs) and lithium-ion batteries. They help to transport ions through a medium, facilitating energy production or storage.
These types include:
Zirconia-based electrolytes
Zirconia-based electrolytes are typically stabilized by grafting in Yttrium oxide or Calium oxide. Yttria-stabilized zirconia (YSZ) is the most widely applied electrolyte for SOFCs due to its high ionic conductivity and thermal stability. Another configuration is calcia-stabilized zirconia, which offers similar properties with reduced ionic conductivity and cation defects that enhance reactivity. These electrolytes are often used for intermediate and high-temperature fuel cells.
Lanthanum gallium oxide (LSGM)
This electrolyte displays high ionic conductivity, which increases with temperature. It offers superior conductivity compared to YSZ at intermediate temperatures. Application of this electrolyte in SOFCs helps to improve performance by diminishing operating temperatures and thereby improving system stability.
S트를 sode
Lai sode ceramits combine sodium ion condictivity with thermal stability and strength properties. Besides this, NASICON can operate at room temperatures, making it suitable for applications that require battery storage systems outside the high-temperature environments.
Comparison of ceramic and polymeric electrolytes
Ceramic and polymeric electrolytes greatly differ in terms of structure and material compromise. Polymer electrolytes meshed ion conductivity with flexibility and can be easily incorporated into lighter battery systems. On the other hand, ceramic electrolytes have higher ionic conductivity, thermal stability, and mechanical strength. This makes them suitable for fuel cells and solid-state batteries operating under high temperatures. The choice of electrolyte is therefore depended on the application and performance requirements.
Solid oxide fuel cells (SOFCs)
Ceramic electrolytes are primarily applied in solid oxide fuel cells. Yttria-stabilized zirconia, preferred for its high ionic conductivity and thermal stability, is applied to SOFCs working under high-temperature ranges (600 to 1000°C). These fuel cells convert chemical energy directly into electric power with high efficiency. The electrochemical reaction within the cells generates clean electric power, with water and heat generation as by-products.
Gas sensors
Ceramic electrolytes are a fundamental part of solid-state gas sensors. These sensors operate by measuring the concentration of target gases (such as oxygen) through ionic conduction changes within the electrolyte. Lanthanum gallium oxide and YSZ are commonly applied in oxygen sensors for their sensitivity and stability at high temperatures. These sensors are applied in industrial processes, automotive systems, and environmental monitoring.
Solid-state batteries
Ceramic electrolytes are used in solid-state batteries to replace liquid electrolytes. By offering improved safety, energy density, and cycle stability, these batteries are a suitable choice for electric vehicles and portable electronics. Lithium lanthanum titanate and Li garnet are among ceramic electrolytes that exhibit high lithium ion conductivity, making them attractive for developing next-generation solid-state batteries.
Electrochemical cells
Ceramic electrolytes like YSZ and LSGM are used in electrolytic cells to oxygen evolve and deposit metals therein. These electrolytes operate under high temperatures and oxidative environments, facilitating ionic but not electronic conduction. Fuel cell systems like Molten carbonate fuel cells (MCFCs) and electrolysis of water, where oxygen ions migrate through the electrolyte, fundamentally apply these electrolytes.
Heat insulation and energy conversion
Ceramic electrolytes are applied in energy conversion systems because of their heat-insulating properties. This leads to energy efficiency improvement during power generation processes. For instance, SOFCs can operate more efficiently when their systems incorporate electrolytes that also act as thermal barriers. Industrial processes benefit from this by reducing energy consumption.
Ionic devices of varistor and capacitor
Ceramic electrolytes are applied in varistors and ceramic capacitors that exhibit electrical insulating and dielectric properties and electrochemical activity. This affects the performance of these devices in high temperatures and challenging environments. In their electronic gadgets, manufacturers leverage enhanced reliability and longer lifespan.
Typically, the ceramic electrolytes can be directly deposited on the electrode materials or embedded within solid-state battery electrode materials during manufacturing. 3D printing, tape casting, and other deposition techniques are used to integrate these electrolytes into devices.
While operating, the ceramic electrolytes allow ions to migrate across their structures during fuel cell operations. In solid-state batteries, they facilitate lithium ions transfer through electrolyte layers electrically insulating against electron flow for safety.
Repairing worn-out or damaged ceramic electrolytes is as simple as replacing the device into which they are integrated. The ionic conduction mechanism, anti-corrosive properties, and mechanical strength of these electrolytes are maintained by working conditions and using compatible materials.
To help preserve the ceramic electrolytes' integrity, fuelling your fuel cells with pure hydrogen and oxygen, using high-purity feedstock, and operating within designed temperature ranges can minimise the likelihood of damage.
Some of the key factors that define fuel cells' quality, reliability, and long life span include:
Material composition
Ceramic electrolyte materials primarily determine ionic conductivity. Yttria-stabilised zirconia made of zirconium oxide and yttrium oxide is a highly conductive electrolyte. It fundamentally achieves exceptional conductivity at elevated temperatures. Lanthanum gallium oxide displays superior room temperature capability to LLTO. The landform of sodium beta alumina contributes to mechanical strength and chemical stability.
Manufacturing process
Using improper techniques can create flaws, crack internal structures, and lead to particle agglomeration. This hinders performance and stability. On the flip side, sophisticated manufacturing techniques like tape casting and 3D printing can produce ceramic electrolytes of high microhomogeneity with superior density and microstructure. Quality put together and process selection fundamentally affect electrolyte quality and the fuel cells' reliability.
Operating conditions
Ceramic electrolytes operate at a range of temperatures between 500°C to 1000°C. They maintain electrochemical stability under harsh operating conditions. Inconsistent temperature gradient, mechanical stress, and chemical aggression can jeopardize electrolyte stability. Operating the fuel cell within the specified range limits prolonged exposure to extreme conditions. Doing this helps improve the electrolyte's durability and increases its lifespan by reducing wear and tear.
Chemical compatibility
Chemical incompatibility between electrolytes and fuels or oxidants leads to electrolyte degradation over time. YSZ is chemically stable in hydrogen, steam, and air but can degrade in carbonate or sulfur-containing species. Lanthanum gallium oxide is stable under reducing and oxidizing atmospheres. Selecting an electrolyte compatible with the fuel cell operating environment improves the electrolyte's integrity and performance over time.
Microstructure
Pores and cracks formed during electrolyte production and particle agglomeration can contribute to the poor quality and stability of electrolytes. They disrupt ionic conduction slow down or halt fuel cell operation. A stable microstructure free of defects promotes uninterrupted ion flow and maintains energy production continuity. Stability is fundamental to operation continuity, and therefore, users have to consider it when purchasing these electrolytes. Stability prolongs cell efficiency by several folds.
Electrolyte design
Recent designs that integrate electrolyte materials with hierarchical porous structures offer fuel cells new ways of improving stability. High stability space occupies within this structure allows ion transport, while porous electrodes improve mass transfer. Designing electroless plating and fuel cell epitaxy help produce stable electrolytes compatible with various operating conditions. These advancements are fundamental in enhancing stability and developing next-generation solid fuel technology.
A1. Ceramic electrolytes are ionic conduction materials. They are used in fuel cells, solid-state batteries, and electrochemical cells. Withstanding elevated temperatures and chemically stable, this electrolyte type offers advantages like enhanced safety, improved efficiency, prolonged operational life, and sustainability. They are preferred for high-performance energy applications.
A2. The benefits of using ceramic electrolytes include improved energy density, enhanced safety, and greater efficiency. They also offer longer lifetimes in solid-state batteries and fuel cells due to chemical stability, overheating, and preventing fires or explosions. Being environmental friendly, they are also sustainable since they have low or no emissions during operation.
A3.Certainly, ceramic electrolytes are designed to operate at elevated temperatures. They typically range from 500°C to 1000°C. Yttria-stabilized zirconia and lanthanum gallium oxide are among electrolytes that can easily tolerate these temperatures. Unique structures and compositions contribute to ionic conduction stability, making them applicable to high-temperature fuel cells and batteries.
A4.Most ceramic electrolytes are rigid and brittle because of their inherent material properties. These electrolytes can be manufactured into thin films that make them easier to integrate into devices. Flexible alternatives, such as polymer-based composites incorporating ceramic particles, do exist but offer a different performance profile—lower stability at temperature and reduced conductivity—compared to pure ceramics.